BOSULIF is a prescription medicine used to treat:
- adults and children 1 year of age and older who have a certain type of leukemia called chronic phase (CP) Philadelphia chromosome–positive chronic myelogenous leukemia (Ph+ CML) who are newly diagnosed or who no longer benefit from or did not tolerate other treatment
- adults with accelerated phase (AP), or blast phase (BP) Ph+ CML who can no longer benefit from or did not tolerate other treatment
It is not known if BOSULIF is safe and effective in children less than 1 year of age with CP Ph+ CML who are newly diagnosed or who no longer benefit from or did not tolerate other treatment or in children with AP Ph+ CML or BP Ph+ CML.
BOSULIF is part of a group of medicines called tyrosine kinase inhibitors (TKIs). TKIs can block BCR-ABL function,
slowing the growth of leukemia cells. For some patients, this allows their white blood cells to return to normal levels.
How Adult Patients with CML Responded to BOSULIF in Clinical Trials
- Results in adult patients who are newly diagnosed
- Results in adult patients who no longer benefit from or did not tolerate other treatment
BOSULIF was studied in adult patients with newly
diagnosed CP Ph+ CML and compared with adult patients
treated with imatinib, which is also a TKI used to treat Ph+ CML.
To evaluate how well BOSULIF worked to reduce
the amount of cancer cells in the blood and bone
marrow, doctors or healthcare professionals (HCPs)
look at the level of major molecular response
(MMR) and complete cytogenetic response (CCyR).
A molecular response measures the BCR-ABL gene in your
blood. MMR is when the amount of BCR-ABL gene in your
blood is low.
A cytogenetic response measures the decrease in the
number of Philadelphia chromosomes in leukemia
cells. CCyR is when no cells with the Philadelphia
chromosome can be found in your bone marrow.
response rates
compared with imatinib
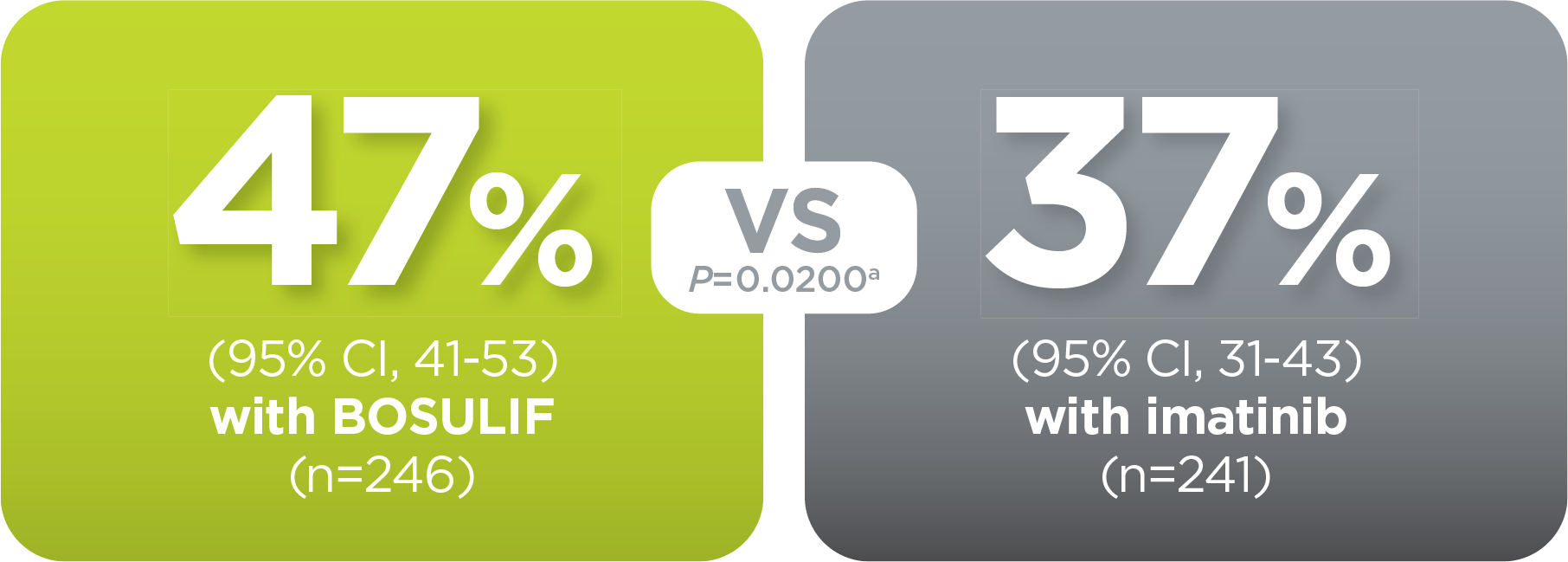
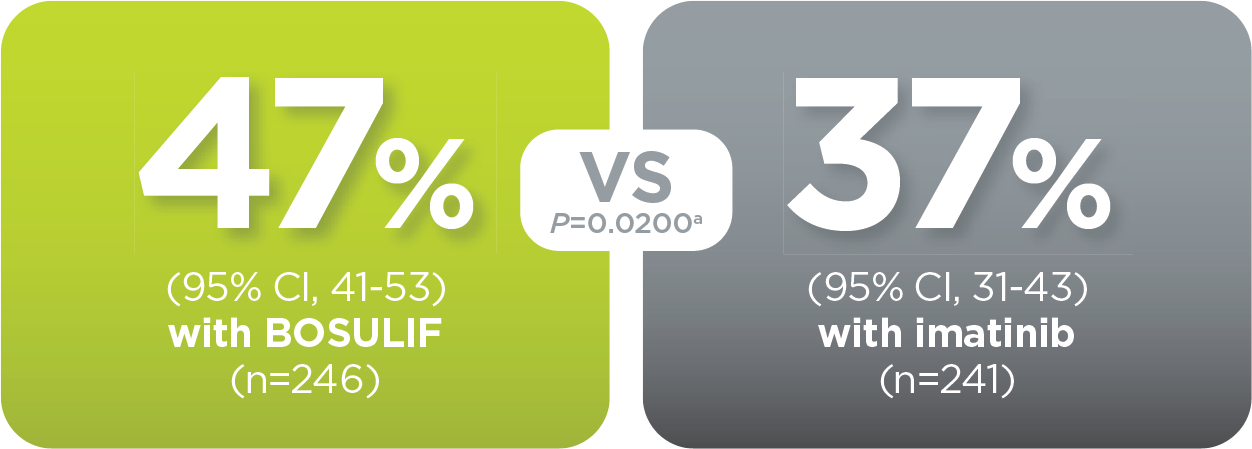
With BOSULIF, more adult patients
achieved MMR at 12 months vs imatinib
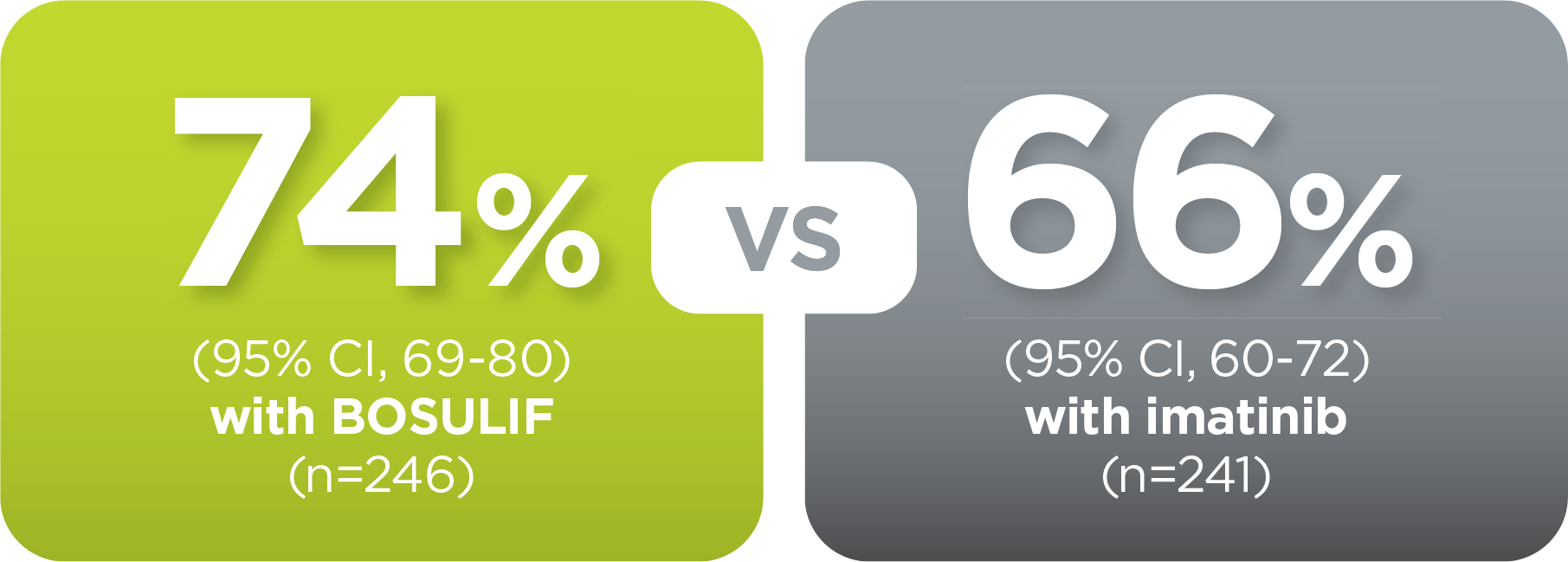
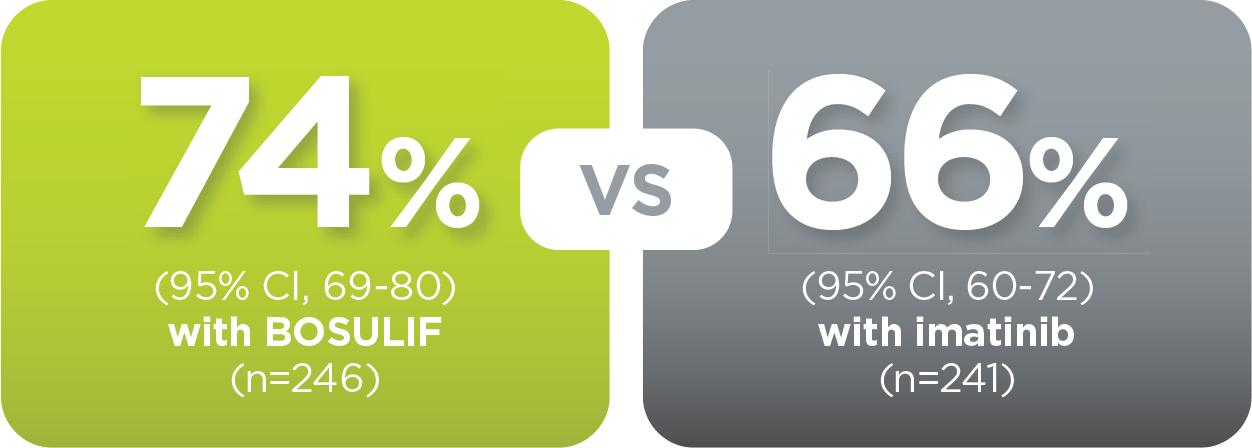
With BOSULIF, more adult patients
achieved MMR at 5 years vs imatinib
BOSULIF was studied in adult patients with Ph+ CML in:
- Chronic phase (CP) who had been treated with 1 prior TKI
- CP who had been treated with 2 or more prior therapies
- Accelerated phase (AP) or blast phase (BP) who had been treated with at least 1 prior TKI
CML Phasesb
Chronic myelogenous leukemia (CML) has 3 phases:

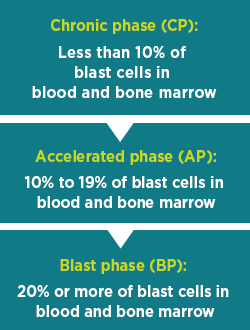
Talk to your doctor about any questions or concerns you may have about the different phases.
To see if BOSULIF worked in the clinical trial of adult patients who no longer benefitted from or did not tolerate other treatment, doctors looked at patients’ blood and bone marrow samples regularly.
In adult patients in chronic phase, efficacy was measured by major cytogenetic response (MCyR), which is when the percentage of leukemia cells with the Philadelphia chromosome is less than or equal to 35%.
Adult patients in CP who had been treated with 1 prior TKI imatinib
- By 6 months from the start of therapy with BOSULIF, 40% (n=105) of these patients had an MCyR
- When these patients were followed for a longer period (at least 60 months), 60% (n=156) of these patients achieved an MCyR at some point during that period
Adult patients in CP who had been treated with 2 or more prior TKIs
- By 6 months from the start of therapy with BOSULIF, 26% (n=29) of these patients had an MCyR
- When these patients were followed for a longer period (at least 48 months), 40% (n=45) of patients achieved an MCyR at some point during that period
95% of adult patients stayed
in CP while taking BOSULIF
5% of adult patients in CP had
confirmed disease transformation
to AP or BP while taking BOSULIF
Adult patients in AP or BP who had been treated with at least 1 prior TKI
- When patients were followed for 48 weeks, 31% (22/72) of patients in AP achieved a CHR at some point during that period
- When patients were followed for 48 weeks, 17% (10/60) of patients in BP achieved a CHR at some point during that period
- When patients were followed for 48 weeks, 57% (41/72) of patients in AP achieved an OHR at some point during that period
- When patients were followed for 48 weeks, 28% (17/60) of patients in BP achieved an OHR at some point during that period
Dealing With Diarrhea
Most patients in the clinical studies of BOSULIF experienced diarrhea. Nine percent of adult patients who were newly diagnosed and 10% of adult patients with CP CML treated before experienced episodes of severe diarrhea.
Before starting BOSULIF, ask your doctor or healthcare professional (HCP) how to prepare for possible episodes of diarrhea.
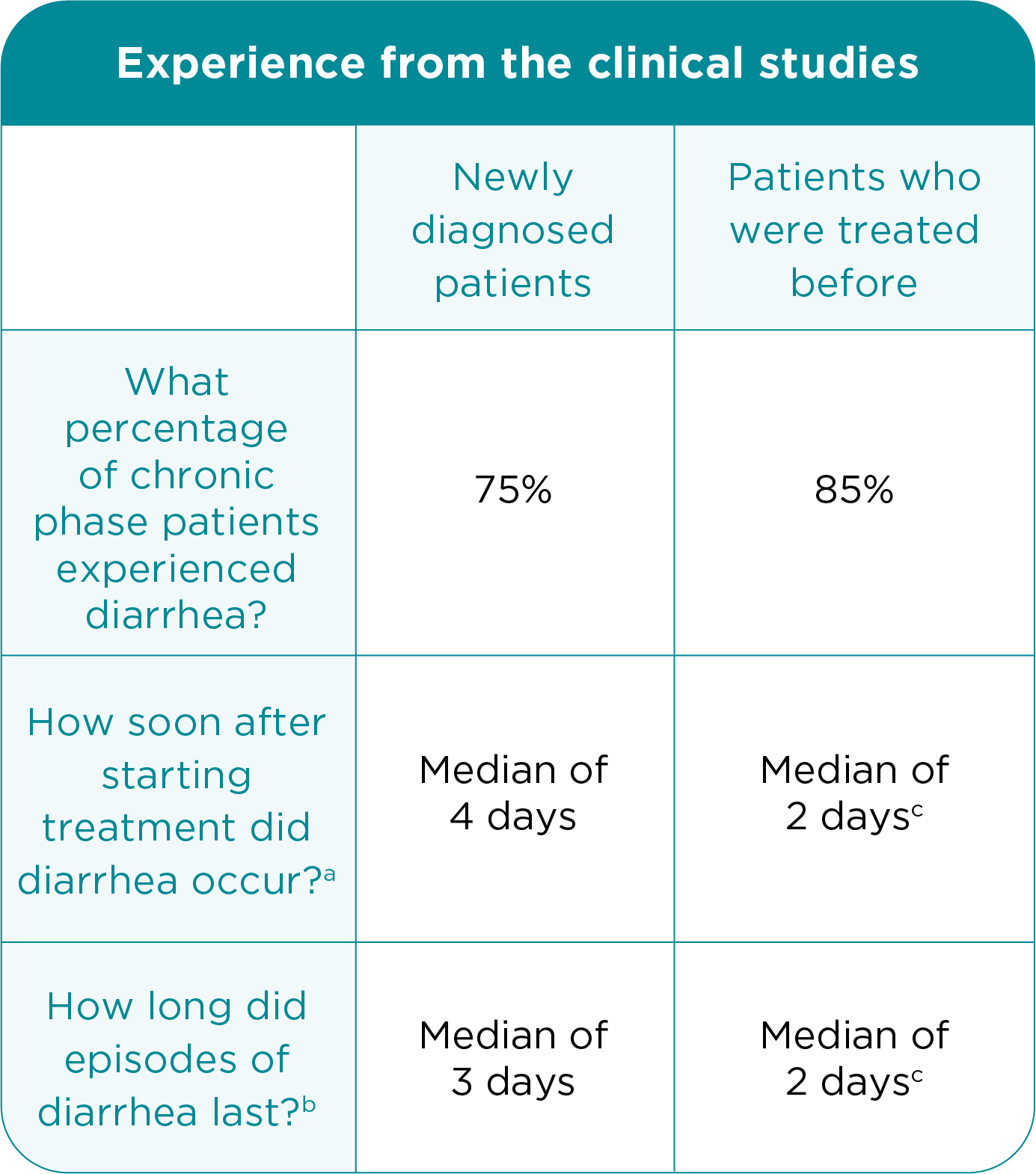
aMedian length of time after starting treatment that diarrhea occurred.
(The median is the “middle value” in a list of numbers. It is a kind of
measurement. For example, the median number of episodes of diarrhea
means that half of the patients in the study experienced more episodes of
diarrhea, and half experienced fewer episodes of diarrhea.)
b Median length of each diarrhea episode.
c Includes advanced phase patients.
What to do if you experience diarrhea
If you have diarrhea, call your doctor or HCP. Your
doctor or HCP may recommend you take
medicine to treat diarrhea. Always talk to your
doctor or HCP before taking any over-the-counter
medicines. Your doctor or HCP may change your
dose,
temporarily stop, or permanently stop
treatment with BOSULIF to help manage diarrhea.
Possible Side Effects
It’s important to be aware of the side effects
so you know what may occur.
including:
- Stomach problems. BOSULIF may cause stomach (abdomen) pain, nausea, diarrhea, vomiting, or blood in your stools. Get medical help right away for any stomach problems
- Low blood cell counts. BOSULIF may
cause low platelet counts
(thrombocytopenia), low red blood
cell counts (anemia), and low white
blood cell counts (neutropenia). Your
doctor should do blood tests
to check your blood cell counts
regularly during your treatment with BOSULIF. Call your doctor right
away if you have unexpected bleeding
or bruising, blood in your urine or
stools, fever, or any signs of an infection
- Liver problems. Your doctor should do
blood tests to check your liver function
regularly during your treatment with
BOSULIF. Call your doctor right away if
your skin or the white part of your
eyes turns yellow (jaundice) or you
have dark “tea color” urine
- Heart problems. BOSULIF may cause heart problems, including heart failure and decreased blood flow to the heart, which can lead to heart attack. Get medical help right away if you get shortness of breath, weight gain, chest pain, or swelling in your hands, ankles, or feet
- Your body may hold too much fluid (fluid retention). Fluid may build up in the lining of your lungs, the sac around your heart, or your stomach cavity. Get medical help right away if you get any of the following symptoms during your treatment with BOSULIF:
- shortness of breath and cough
- swelling in your hands, ankles, or feet
- weight gain
- chest pain
- swelling all over your body
- Kidney problems. Your doctor should do tests to check your kidney function when you start treatment with BOSULIF and during your treatment. Call your doctor right away if you get any of the following symptoms during your treatment with BOSULIF:
- you urinate more or less often than normal
- you make a much larger or smaller amount of urine than normal
The most common side effects of BOSULIF in adults and children with CML include diarrhea, stomach (abdominal)
pain, vomiting, nausea, rash, tiredness, liver problems, headache, fever, decreased appetite, respiratory tract infections
(infections in nose, throat, or lungs), constipation, and changes in certain blood tests. Your doctor may do blood tests
during treatment with BOSULIF to check for changes.
Tell your doctor or get medical help right away if you get respiratory tract infections, loss of appetite, headache,
dizziness, back pain, joint pain, rash, or itching while taking BOSULIF. These may be symptoms of a severe allergic reaction.
Your doctor may change your dose, temporarily stop, or permanently stop treatment with BOSULIF if you
have certain side effects.
BOSULIF may cause fertility problems in both female and male patients. This may affect your ability to have a child.
Talk to your doctor if this is a concern for you.
Tell your doctor if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of BOSULIF. For more information, ask your doctor or pharmacist. You may report side effects to FDA at 1-800-FDA-1088.
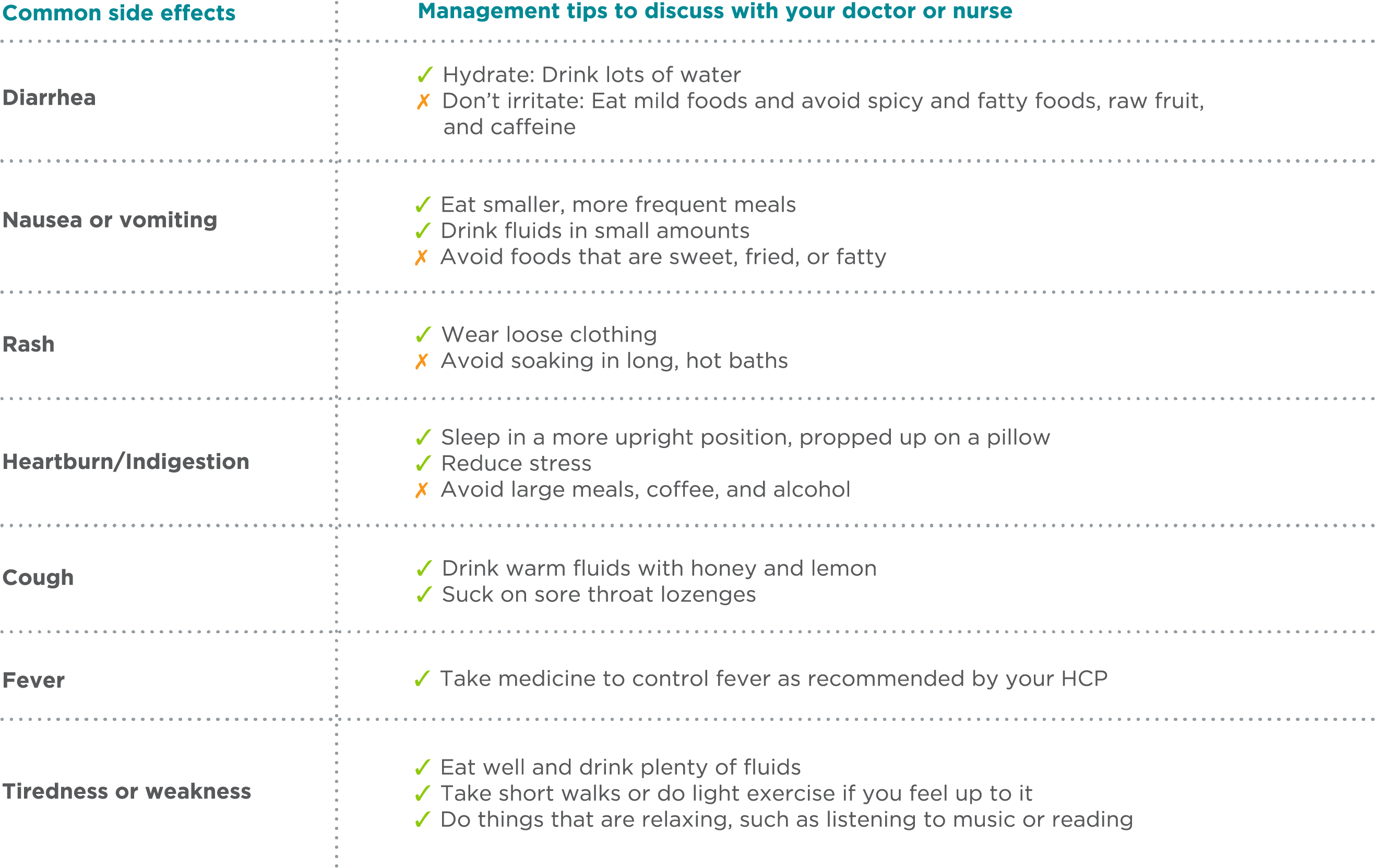
Management Tips for Potential Side Effects
The table below contains information about some common side effects of BOSULIF and tips that may help manage them. It’s important to discuss this content with your doctor or HCP and set up a plan for managing any side effects you may have. Ask your doctor or HCP if there are over-the-counter or prescription medicines that may help you. Not all side effects are manageable. Your doctor may change your dose or tell you to stop taking BOSULIF.
QUICK TIP: Track How You’re Feeling on the MyTherapy App
Track how you’re feeling so that, together you and your healthcare team can set up a plan for managing any side effects you may have.





